
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Novartis Announces Positive Results For Cardiovascular Drug

Novartis AG (NYSE:NVS) announced positive top-line results from the global phase III study, CANTOS. The study evaluated the efficacy, safety and tolerability of ACZ885 (canakinumab) in combination with standard of care in patients with a prior heart attack and inflammatory atherosclerosis.
CANTOS is a randomized, double-blind, placebo-controlled, event-driven phase III study. It is designed to evaluate the efficacy, safety and tolerability of quarterly subcutaneous injections of canakinumab in combination with standard of care in the prevention of recurrent cardiovascular (CV) events. The study enrolled patients with a prior myocardial infarction (MI) and with a high-sensitivity C-reactive protein (hsCRP).
The study met the primary endpoint as the candidate demonstrated canakinumab when used in combination with standard of care reduced the risk of major adverse cardiovascular events (MACE). Novartis will present the full data later in 2017.
Approximately 580,000 people in the EU and 750,000 people in the U.S. are affected by heart attacks every year. Heart patients remain susceptible to a second attack and even death. 25% of heart attack survivors are estimated to experience another cardiovascular event within five years. Among four in 10 patients, the risk is directly related to increased inflammation associated with atherosclerosis thereby highlighting the great need for treatments like canakinumab.
The successful development and commercialization of canakinumab will significantly boost Novartis’ cardiovascular portfolio. The company currently has Entresto in its portfolio which has been given a Class I recommendation in the U.S. and EU clinical guidelines for the treatment of heart failure with reduced ejection fraction (HFrEF).
We remind investors that canakinumab was first approved in 2009 for cryopyrin-associated periodic syndromes as Ilaris. In 2016, the FDA approved the label expansion of the drug to treat three rare and distinct types of periodic fever syndromes – tumor necrosis factor-receptor associated periodic syndrome, hyperimmunoglobulin D syndrome / mevalonate kinase deficiency and familial Mediterranean fever.
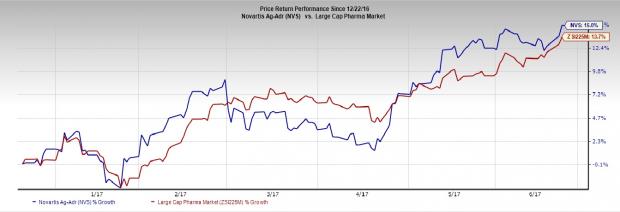
Novartis has outperformed the Zacks classified industry in the last six months. The stock has rallied 15.0% compared with the Large Cap Pharmaceuticals industry’s gain of 13.7%.
Going forward, we expect that the approval of new drugs and label expansion of existing ones will bode well for Novartis even though stiff generic competition for key drugs like Gleevec continues to act as a major deterrent. Earlier in the week,
Novartis announced that that its generic arm, Sandoz, has received European Commission (EC) approval for Rixathon, a biosimilar version of Roche’s (OTC:RHHBY) MabThera in Europe. The FDA accepted Sandoz’s Abbreviated New Drug Application (ANDA) for a generic version of GlaxoSmithKline’s (NYSE:GSK) asthma drug, Advair Diskus.
Zacks Rank
Novartis currently carries a Zacks Rank #3 (Hold). A better-ranked stock in healthcare sector include VIVUS, Inc. (NASDAQ:VVUS) which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
VIVUS’s loss per share estimates lessened from 50 cents to 39 cents for 2017 in the last 60 days. The company posted positive earnings surprises in all four trailing quarters with average beat of 233.69%
Looking for Ideas with Even Greater Upside?
Today's investment ideas are short-term, directly based on our proven 1 to 3 month indicator. In addition, I invite you to consider our long-term opportunities. These rare trades look to start fast with strong Zacks Ranks, but carry through with double and triple-digit profit potential. Starting now, you can look inside our home run, value, and stocks under $10 portfolios, plus more. Click here for a peek at this private information >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
GlaxoSmithKline PLC (GSK): Free Stock Analysis Report
VIVUS, Inc. (VVUS): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Alibaba (NYSE:BABA) are on a tear to start off 2025. The consumer discretionary and tech stock is up by 52% this year as of the Feb. 25 close. The company’s cloud...

Every investor should know the term CEP, or customer engagement platform, because it is central to businesses' use of AI. CEPs provide software services to connect and communicate...

As markets try to look through the blizzard of policy changes flowing out of Washington, the crowd has shifted its preferences considerably in recent weeks based on a sector lens....
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.