Aduro Biotech, Inc. (NASDAQ:ADRO) announced that the FDA has cleared the Investigational New Drug application (IND) for its pipeline candidate ADU-S100 (MIW815). This candidate will be evaluated in combination with Novartis’ (NYSE:NVS) investigational anti-PD-1 checkpoint inhibitor- PDR001, for the treatment of advanced/metastatic solid tumors or lymphomas.
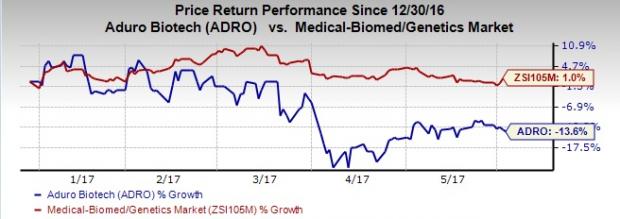
Aduro’s shares underperformed the Zacks classified Medical-Biomedical and Genetics industry year to date. Shares of Aduro declined 13.6% during the period, while the industry witnessed an increase of 1%.
Moving ahead, Aduro, an immunotherapy company, in collaboration with Novartis is also planning to initiate a phase Ib trial of ADU-S100 in combination with anti-PD1 in the second half of 2017. The phase Ib trial is designed to assess the safety and efficacy of ADU-S100 administered by intratumoral injection with PDR001 to patients with advanced/metastatic solid tumors or lymphomas.
We remind investors that in May 2016, Aduros initiated a phase I, multicenter, dose escalation study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and anti-tumor activity of ADU-S100 in patients with cutaneously accessible metastatic solid tumors or lymphomas. This study is currently ongoing and the company expects to expand into viscerally accessible lesions in the second half of 2017.
We also remind investors that in Mar 2017, Merck & Co., Inc. ‘s (NYSE:MRK) anti-PD-1 therapy, Keytruda, received positive opinion from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) for the treatment of patients with relapsed or refractory classical hodgkin lymphoma (cHL).
Zacks Rank and Stocks to Consider
Aduro carries a Zacks Rank #3 (Hold). A better-ranked stock in the health care sector includes VIVUS, Inc. (NASDAQ:VVUS) sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
VIVUS’ loss per share estimates narrowed from 50 cents to 39 cents for 2017 over the last 30 days. The company posted positive earnings surprises in all of the four trailing quarters, with an average beat of 233.69%.
Will You Make a Fortune on the Shift to Electric Cars?
Here's another stock idea to consider. Much like petroleum 150 years ago, lithium power may soon shake the world, creating millionaires and reshaping geo-politics. Soon electric vehicles (EVs) may be cheaper than gas guzzlers. Some are already reaching 265 miles on a single charge. With battery prices plummeting and charging stations set to multiply, one company stands out as the #1 stock to buy according to Zacks research. It's not the one you think. See This Ticker Free >>
Novartis AG (NVS): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
VIVUS, Inc. (VVUS): Free Stock Analysis Report
Aduro Biotech, Inc. (ADRO): Free Stock Analysis Report
Original post
Zacks Investment Research