
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Mylan Announces IPR Proceedings For Sanofi's Lantus Patents

Mylan N.V. (NASDAQ:MYL) announced that the U.S. Patent and Trademark Appeal Board (“PTAB”) has instituted inter partes review (“IPR”) proceedings on all claims against two Orange Book-listed patents — U.S. Patent Nos. 7,476,652 and 7,713,930, respectively.
The two patents are owned by Sanofi (NYSE:SNY) for Lantus (insulin glargine injection) 100 Units/mL.
We remind investors that Mylan's 505(b)(2) new drug application (“NDA”) for Insulin Glargine in vial and pen dosage forms is under active review with the FDA.
In October, Sanofi initiated a patent infringement litigation against Mylan's NDA in the United States District Court for the District of New Jersey alleging violation of 18 patents in the suit inclusive of these two Lantus patents.
Lantus and Lantus Solostar are both approved for improving glycemic control in adult patients with diabetes mellitus.
Sanofi’s diabetes franchise seems highly weighed on with its key product Lantus facing a tremendous competitive pressure at the payor level as well as from other biosimilars in several European markets besides Japan. Moreover, a biosimilar version of Lantus had hit the markets last December.
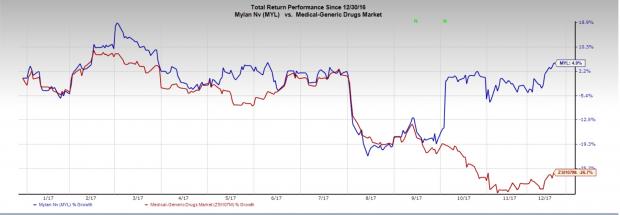
Meanwhile, shares of Mylan have rallied 4.9% so far this year, outperforming the industry’s 26.7% decline during the period.
Mylan is a leading generic drug company in terms of both total and new prescriptions. The company’s pursuit of first-to-file opportunities should help it maintain a strong position in the global generics market.
Mylan is also exploring the world of biosimilars, a market with potential to grow to $20 billion by 2020. A partnership with Biocon and collaborations with Momenta Pharmaceuticals, Inc. (NASDAQ:MNTA) and Mabion have aided the company to develop a portfolio of 16 biosimilar/insulin analog generic products.
However, Mylan’s performance in 2017 continues to be affected by the ongoing challenges in North America. The third quarter witnessed a fast decline in EpiPen sales, thanks to the launch of an authorized generic as well as the contraction of overall epinephrine auto-injector market.
In a major boost, Mylan also received an FDA approval for the generic version of Copaxone 40mg. As one of the first filers, the company will enjoy 180 days of exclusivity. The recent FDA approval for Mylan’s biosimilar version of Roche Holdings’ (OTC:RHHBY) Herceptin will also boost the company’s biosimilar portfolio.
Zacks Rank
Mylan carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Investor Alert: Breakthroughs Pending
A medical advance is now at the flashpoint between theory and realization. Billions of dollars in research have poured into it. Companies are already generating substantial revenue, and even more wondrous products are in the pipeline.
Cures for a variety of deadly diseases are in sight, and so are big potential profits for early investors. Zacks names 5 stocks to buy now.
Click here to see them >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Sanofi (SNY): Free Stock Analysis Report
Momenta Pharmaceuticals, Inc. (MNTA): Free Stock Analysis Report
Mylan N.V. (MYL): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Caesars Entertainment (NASDAQ:CZR), a leading gambling stock, traded around 3% higher on Wednesday morning, though the stock was trading around 1.5% lower shortly before...

Amazon (NASDAQ:AMZN) is making a significant push into the future with a robust investment in robotics and artificial intelligence. The company has earmarked $35 billion for...

Home Depot’s (NYSE:HD) Q4 2024 report and guidance for 2025 have plenty to be unhappy about, but the simple truth is that this company turned a corner in 2024. It is on track for...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.