
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Merck's Keytruda Misses Primary End Point For Gastric Cancer

Merck (NYSE:MRK) announced disappointing results from a pivotal phase III KEYNOTE-061 study, evaluating its anti-PD-1 therapy, Keytruda (pembrolizumab), as a second-line treatment for patients with advanced gastric or gastroesophageal junction (“GEJ”) adenocarcinoma.
Notably, Keytruda is already approved in the United States as a monotherapy for third-line treatment of advanced gastric or GEJ. Additionally, it is approved for many types of cancers and treatment settings including lung cancer, melanoma, head and neck cancer, classical hodgkin lymphoma and bladder cancer.
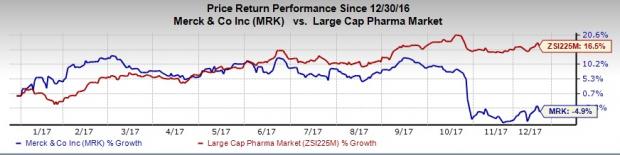
Shares of Merck has lost 4.9% this year so far compared unfavorably with the industry’s 16.5% increase.
The phase III study failed to meet its primary endpoint of overall survival (OS). Notably, the progression free survival (PFS) showed no statistical significance in the PD-L1 positive patient population.
However, the company said that it will continue to evaluate Keytruda in two other phase III studies, both as a monotherapy as well as in combination with chemotherapy as a first-line treatment for patients with GEJ.
We remind investors that Keytruda is a key top-line driver for Merck and it brought in $1.5 billion sales in third-quarter 2017, up 18.8% sequentially and 194% year over year. Sales continue to be driven by a launch of new indications globally.
Significantly, Keytruda sales in the United States have gained particularly from a strong momentum in the new indication of first-line lung cancer. The drug’s sales were primarily driven by melanoma outside the United States.
Meanwhile, the Keytruda development program also significantly advanced this year with regulatory approvals for four new indications in the United States and two additional diseases in Europe.
Important approvals include nods for advanced bladder cancer in both the United States and the EU, advanced microsatellite instability-high cancers and the first sanction for a combination therapy with Eli Lilly & Company’s (NYSE:LLY) cancer drug Alimta (pemetrexed) and carboplatin (pem/carbo), a commonly used chemo regimen in lung cancer.
Keytruda is continuously growing and expanding into new indications and markets globally. It is under trial for more than 30 types of cancer in above 650 studies including excess of 300 combination studies.
Merck is collaborating separately with several companies namely Amgen, Inc. (NASDAQ:AMGN) , Incyte, Glaxo and Pfizer, Inc. (NYSE:PFE) for the evaluation of Keytruda in combination with other regimens.
Zacks Rank
Merck carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Investor Alert: Breakthroughs Pending
A medical advance is now at the flashpoint between theory and realization. Billions of dollars in research have poured into it. Companies are already generating substantial revenue, and even more wondrous products are in the pipeline.
Cures for a variety of deadly diseases are in sight, and so are big potential profits for early investors. Zacks names 5 stocks to buy now.
Pfizer, Inc. (PFE): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Caesars Entertainment (NASDAQ:CZR), a leading gambling stock, traded around 3% higher on Wednesday morning, though the stock was trading around 1.5% lower shortly before...

Amazon (NASDAQ:AMZN) is making a significant push into the future with a robust investment in robotics and artificial intelligence. The company has earmarked $35 billion for...

Home Depot’s (NYSE:HD) Q4 2024 report and guidance for 2025 have plenty to be unhappy about, but the simple truth is that this company turned a corner in 2024. It is on track for...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.