
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Merck's Keytruda Gets FDA Priority Review For Rare Lymphoma

Merck (NYSE:MRK) announced that the FDA has granted priority review to its supplemental biologics license application (sBLA) looking for label expansion of its marketed drug, Keytruda (pembrolizumab), for a rare lymphoma indication.
The sNDA is looking to expand Keytruda’s label for treatment of adult and pediatric patients with refractory primary mediastinal B-cell lymphoma (PMBCL), a type of non-Hodgkin lymphoma (“NHL”). With the FDA granting a priority review, its response is expected on Apr 3, 2018. If finally approved, this would be the second nod for Keytruda from the regulatory body for treating a hematologic malignancy indication.
In March/April, Keytruda was approved both in the United States as well as the EU for refractory classical Hodgkin lymphoma, the first Keytruda approval for hematologic malignancy indication.
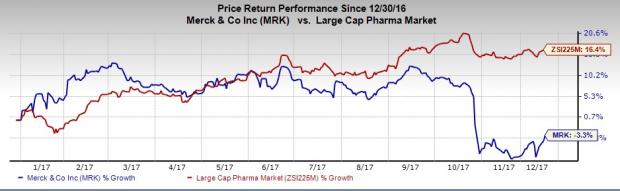
Merck’s shares have declined 3.3% this year so far, underperforming the industry’s16.4% rally.
The sBLA was accepted by the FDA based on encouraging data from the ongoing phase II KEYNOTE-170 and the phase Ib KEYNOTE-013 studies. Both trials are evaluating the safety, tolerability and efficacy of Keytruda as a monotherapy on patients with various blood cancers.
In the same press release, the company released outcomes from the KEYNOTE-170 study. Data from the study demonstrated that patients with a relapsed/refractory PMBCL achieved an overall response rate of 41%. Additionally, 24% of patients achieved a complete response rate and a 17% partial response rate at median follow-up of 10.5 months. The drug was however, unable to achieve the median duration of response. Finds from the study were presented at the 59th annual meeting of American Society of Hematology.
Notably, Keytruda was granted a Breakthrough Therapy Designation by the FDA in January for the aforementioned indication.
Per the company’s press release, PMBCL accounts for an almost 2-4% of all NHL in the United States. Hence, approval of the drug for this expanded indication will provide the company with access to a wider patient population with PMBCL.
We remind investors that Keytruda is the first anti-PD-1 therapy to gain an FDA approval and is being studied for more than 30 types of cancer in above 650 studies including 400 plus combination trials. Key recent approvals of drugs have been given for indications including advanced bladder cancer, advanced microsatellite instability-high cancers and the first approval as a combination therapy was granted with Eli Lilly's (NYSE:LLY) cancer drugs Alimta (pemetrexed) and carboplatin (pem/carbo) for lung cancer.
Zacks Rank & Key Picks
Merck carries a Zacks Rank #3 (Hold). Two better-ranked stocks in the health care sector are Corcept Therapeutics Inc. (NASDAQ:CORT) and Achillion Pharmaceuticals, Inc. (NASDAQ:ACHN) , both carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Corcept’s earnings per share estimates have moved up from 77 cents to 88 cents for 2018 over the last 60 days. The company delivered positive earnings surprises in two of the trailing four quarters with an average beat of 14.32%. Share price of the company has skyrocketed 137.5% year to date.
Achillion’s loss per share estimates has narrowed from 66 cents to 63 cents for 2017 and from 75 cents to 67 cents for 2018 over the last 60 days. The company came up with positive earnings surprises in two of the last four quarters with an average beat of 4.51%.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Eli Lilly and Company (LLY): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Achillion Pharmaceuticals, Inc. (ACHN): Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Alibaba (NYSE:BABA) are on a tear to start off 2025. The consumer discretionary and tech stock is up by 52% this year as of the Feb. 25 close. The company’s cloud...

Every investor should know the term CEP, or customer engagement platform, because it is central to businesses' use of AI. CEPs provide software services to connect and communicate...

As markets try to look through the blizzard of policy changes flowing out of Washington, the crowd has shifted its preferences considerably in recent weeks based on a sector lens....
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.