
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Alnylam Submits MAA In Europe For RNAi Therapeutic Givosiran

Alnylam Pharmaceuticals, Inc. (NASDAQ:ALNY) announced that it has submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) for givosiran, an investigational RNAi therapeutic targeting aminolevulinic acid synthase 1 (ALAS1), in development for the treatment of acute hepatic porphyria (AHP).
The MAA was supported by data from the pivotal ENVISION phase III study. In the study, givosiran met the primary efficacy endpoint, with a 74% mean reduction relative to placebo in the annualized rate of composite porphyria attacks. There was a corresponding 90% median reduction in composite annualized attack rate (AAR), with a median AAR of 1.0 in givosiran patients compared with that of 10.7 in placebo patients. In the trial, 50% of givosiran-treated patients were attack free during the six-month treatment period compared to 16.3% of placebo-treated patients.
Givosiran previously received Breakthrough Therapy designation and Orphan Drug status from the FDA for AHP. The drug has also been granted Priority Medicines (PRIME) designation and Orphan Drug status by the EMA for the same.
In June 2019, the company also completed the rolling submission of a new drug application (NDA) to the FDA for givosiran in AHP.
Shares of Alnylam have declined 1.3% year to date against the industry’s growth of 7.4%.
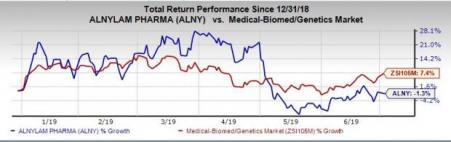
We remind investors that Onpattro, a first-of-its-kind RNAi therapeutic, is the company’s only approved drug. It is also the only FDA-approved drug for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults.
The company also has other candidates in its pipeline. Alnylam along with partner The Medicine Company (NASDAQ:MDCO) is evaluating inclisiran in phase III ORION studies for hypercholesterolemia.
Alnylam’s expertise in RNAi therapeutics and broad intellectual property estate have allowed it to enter collaborations with leading pharmaceutical and life sciences companies, including Ionis Pharmaceuticals, Novartis (NYSE:NVS) , Roche (OTC:RHHBY) , Takeda, Merck and Sanofi’s specialty care global business unit, Genzyme, among others.
Zacks Rank
Alnylam currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
This Could Be the Fastest Way to Grow Wealth in 2019
Research indicates one sector is poised to deliver a crop of the best-performing stocks you'll find anywhere in the market. Breaking news in this space frequently creates quick double- and triple-digit profit opportunities.
These companies are changing the world – and owning their stocks could transform your portfolio in 2019 and beyond. Recent trades from this sector have generated +98%, +119% and +164% gains in as little as 1 month.
Click here to see these breakthrough stocks now >>
Novartis AG (NVS): Free Stock Analysis Report
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
The Medicines Company (MDCO): Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Alibaba (NYSE:BABA) are on a tear to start off 2025. The consumer discretionary and tech stock is up by 52% this year as of the Feb. 25 close. The company’s cloud...

Every investor should know the term CEP, or customer engagement platform, because it is central to businesses' use of AI. CEPs provide software services to connect and communicate...

As markets try to look through the blizzard of policy changes flowing out of Washington, the crowd has shifted its preferences considerably in recent weeks based on a sector lens....
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.