
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Gilead To Submit NDA For Inflammation Drug Filgotinib In '19

Gilead Sciences (NASDAQ:GILD) announced that it is planning to submit a new drug application (“NDA”) seeking approval for its oral JAK1 inhibitor, filgotinib, as a treatment for rheumatoid arthritis (“RA”) in 2019. The decision was taken by the company following a pre-NDA meeting with the FDA.
The NDA will likely include data from the ongoing phase III clinical program, FINCH, which comprises three studies. The company had discussion on the phase III FINCH studies with the regulatory authority during the pre-NDA meeting. In 2016, the company had collaborated with Galapagos (NASDAQ:GLPG) for the development and commercialization of filgotinib, for inflammatory disease indications including RA.
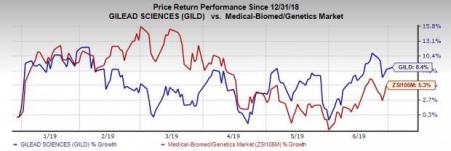
Shares of Gilead have increased 8.4% so far this year compared with the industry’s rise of 5.3%.
The 52-week phase III study, FINCH 1 evaluated filgotinib in comparison with AbbVie’s (NYSE:ABBV) Humira (adalimumab) or placebo on a stable background dose of methotrexate (“MTX”) in patients with prior inadequate response to methotrexate. In March, the company announced 24-week data from the study. The study achieved its primary endpoint for both doses of filgotinib — 100 mg and 200 mg. Filgotinib demonstrated significantly higher ACR20/50/70 responses compared to placebo in patients with prior inadequate methotrexate response. The higher dose of filgotinib also demonstrated non-inferiority to Humira.
The company also announced 24-week data from the ongoing phase III FINCH 3 study in the same month. The study evaluated 100mg or 200mg doses of filgotinib in combination with MTX and as monotherapy in MTX-naive patients. Both the doses of filgotinib in combination with MTX demonstrated significantly higher ACR20/50/70 responses than methotrexate alone. The study achieved its primary endpoint, as higher percentage of patients achieved the primary endpoint of ACR20 response at week 24 for filgotinib 200 mg plus MTX and filgotinib 100 mg plus MTX compared with MTX alone.
In September 2018, Gilead announce top-line data from the phase FINCH 2 study, which evaluated filgotinib in patients receiving conventional synthetic disease-modifying anti-rheumatic drug (DMARD) and prior inadequate response/intolerance to biologic DMARD. The study achieved its primary endpoint in the proportion of patients achieving an American College of Rheumatology 20% response (ACR20) at week 12. Both the doses — 100 mg and 200 mg — achieved significantly higher ACR20/50/70 responses than placebo in patients with active rheumatoid arthritis and prior inadequate response to biologic agents.
Meanwhile, interim safety data from the phase III FINCH program along with updated week 156 safety data from the phase IIb DARWIN 3 long-term extension study showed that the safety profile of the candidate was consistent with previous studies.
The FINCH studies are among several clinical trials of filgotinib in inflammatory diseases including ulcerative colitis.
Gilead is looking to expand into HIV and newer avenues to boost its top line and offset the loss of sales in HCV products. A potential approval to filgotinib for RA and successful development in other inflammation indications are expected to favorably impact the company’s revenues as the targeted indication has significant market opportunity. We note that Eli Lilly (NYSE:LLY) received approval for Olumiant as a treatment for RA last year. Several other pharma companies are developing their drugs for inflammation indications including RA.
Zacks Rank
Gilead currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
This Could Be the Fastest Way to Grow Wealth in 2019
Research indicates one sector is poised to deliver a crop of the best-performing stocks you'll find anywhere in the market. Breaking news in this space frequently creates quick double- and triple-digit profit opportunities.
These companies are changing the world – and owning their stocks could transform your portfolio in 2019 and beyond. Recent trades from this sector have generated +98%, +119% and +164% gains in as little as 1 month.
Click here to see these breakthrough stocks now >>
AbbVie Inc. (ABBV): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Galapagos NV (GLPG): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Alibaba (NYSE:BABA) are on a tear to start off 2025. The consumer discretionary and tech stock is up by 52% this year as of the Feb. 25 close. The company’s cloud...

Every investor should know the term CEP, or customer engagement platform, because it is central to businesses' use of AI. CEPs provide software services to connect and communicate...

As markets try to look through the blizzard of policy changes flowing out of Washington, the crowd has shifted its preferences considerably in recent weeks based on a sector lens....
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.