
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Lilly's Verzenio Gets Priority Review In First-Line Setting

Eli Lilly and Company (NYSE:LLY) announced that the FDA has granted priority review to its new drug application (NDA) for Verzenio (abemaciclib). This drug is looking forward to get approved for the first-line treatment of advanced breast cancer.
Markedly, the NDA was based upon the positive interim results from MONARCH 3 study. The study compared abemaciclib in combination with an aromatase inhibitor versus an aromatase inhibitor alone as initial endocrine-based therapy in patients with HR+, HER2- advanced breast cancer. A decision from the FDA is expected by June 2018.
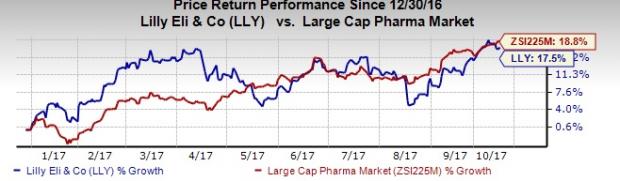
So far this year, Lilly’s shares have underperformed the industry. While the stock has been up 17.5%, the industry gained 18.9%.
Generally, Priority Review designation from the FDA is granted to drugs that have the potential to provide significant improvements in the safety and effectiveness of the treatment, prevention or diagnosis of a serious disease.
Verzenio, a CDK 4/6 inhibitor, is one such drug that was approved by the FDA last week for two breast cancer indications. The drug got approved as a monotherapy for the treatment of hormone-receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer, who had prior endocrine therapy and chemotherapy for metastatic disease and as a combination therapy with fulvestrant in HR+, HER2- advanced breast cancer patients, who had disease progression following endocrine therapy. While the monotherapy submission was based on data from the MONARCH 1 study, the combination application was based on data from the MONARCH 2 study.
We remind the investors that Novartis AG’s (NYSE:NVS) Kisqali, is already approved by the FDA. The drug is used in combination with an aromatase inhibitor for the first-line treatment of postmenopausal women with HR+/HER2- advanced or metastatic breast cancer.
Also, AstraZeneca, plc’s (NYSE:AZN) breast cancer drug, Faslodex, received the FDA approval in August for a label extension in the first-line monotherapy setting for advanced breast cancer.
Zacks Rank & Stock to Consider
Lilly carries a Zacks Rank #3 (Hold). A better-ranked stock in the health care sector includes ACADIA Pharmaceuticals Inc. (NASDAQ:ACAD) carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Over the last 60 days, ACADIA’s loss per share estimates narrowed from $2.63 to $2.52 for 2017 and from $1.92 to $1.85 for 2018. The company delivered positive earnings surprises in two of the trailing four quarters, with an average beat of 7.97%. The share price of the company increased 23% year to date.
4 Stocks to Watch after the Massive Equifax (NYSE:EFX) Hack
Cybersecurity stocks spiked on recent news of a data breach affecting 143 million Americans. But which stocks are the best buy candidates right now? And what does the future hold for the cybersecurity industry?
Equifax is just the most recent victim. Computer hacking and identity theft are more common than ever. Zacks has just released Cybersecurity! An Investor’s Guide to inform Zacks.com readers about this $170 billion/year space. More importantly, it highlights 4 cybersecurity picks with strong profit potential.
Get the new Investing Guide now>>
Astrazeneca (LON:AZN) PLC (AZN): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Nvidia’s earnings beat didn’t erase investor concerns over slowing growth. Soft Q1 guidance and valuation worries may limit the stock’s upside. Weak network and gaming sales...

Shares of Etsy (NASDAQ:ETSY) are down approximately 7% since the company reported earnings on February 19. Concerns over slowing growth are overriding revenue and earnings that...

The price-to-earnings (P/E) ratio is one of the most commonly used metrics to determine whether a stock is expensive or cheap. Generally, the higher the P/E ratio, the more...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.