
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Bristol-Myers (BMY) Reports Positive Data On Leukemia Drug

Bristol-Myers Squibb Company (NYSE:BMY) announced positive data from phase II study, CA180-372, on Sprycel.
Patients received Sprycel 60 mg/m2tablets or powder for oral suspension, once daily, in addition to a chemotherapy regimen modelled on a Berlin-Frankfurt-Munster high-risk backbone for two years or until the occurrence of unacceptable toxicity in the ongoing CA180-372 (NCT01460160) study.
The study evaluated Sprycel in pediatric patients with newly diagnosed Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) when added to a chemotherapy regimen. The combination demonstrated an event-free survival rate, the study’s primary endpoint, of 65.5% and an overall survival rate of 91.5% after three years.
Meanwhile, patients who had minimal residual disease (MRD) ≥0.05% at the end of the first block of treatment and those with MRD 0.005-0.05% who remained MRD-positive at any detectable level after three additional high-risk chemotherapy blocks, were eligible for hematopoietic stem cell transplantation (HSCT) in first remission.
We remind investors that Sprycel was approved in the United States for the treatment of adults with Ph+ chronic myeloid leukemia (CML) in chronic phase (CP) who are resistant or intolerant to prior therapy including imatinib. Sprycel was also approved for adults with Ph+ ALL who are resistant or intolerant to prior therapy.
The drug is also approved for the treatment of adults with newly diagnosed CP Ph+ CML. Sprycel received FDA approval for the expanded indication for treatment in pediatric patients with CP Ph+ CML in November 2017.
The label expansion should improve sales further.
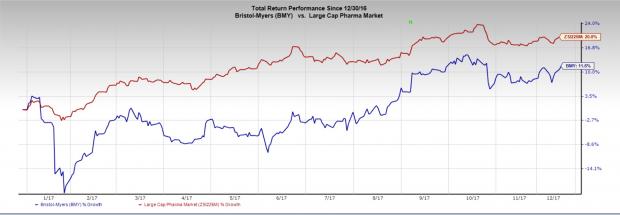
So far this year, Bristol-Myers’ shares have rallied 11.6% compared with the industry's gain of 20%.
Bristol-Myers’ immuno-oncology Opdivo continues to perform well along with Eliquis and Orencia. The company is looking to expand Opdivo’s label further and recently obtained FDA approval for liver and colorectal cancer. However, Opdivo is currently facing competitive challenges in the United States.
The virology business is also under pressure. The company is also looking to counter generic threat for key drugs through deals and acquisitions. The company recently entered into a deal with AbbVie, Inc. (NYSE:ABBV) and Halozyme Therapeutics, Inc. (NASDAQ:HALO) .
Zacks Rank & Stock to Consider
Bristol-Myers currently carries a Zacks Rank #3 (Hold).
A better-ranked health care stocks is Sucampo Pharmaceuticals (NASDAQ:SCMP) with a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Sucampo’s earnings per share estimates have moved up from 97 cents to $1.19 for 2018 over the last 30 days. The company delivered a positive earnings surprise in three of the trailing four quarters, with an average beat of 15.63%.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Halozyme Therapeutics, Inc. (HALO): Free Stock Analysis Report
Sucampo Pharmaceuticals, Inc. (SCMP): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Alibaba (NYSE:BABA) are on a tear to start off 2025. The consumer discretionary and tech stock is up by 52% this year as of the Feb. 25 close. The company’s cloud...

Every investor should know the term CEP, or customer engagement platform, because it is central to businesses' use of AI. CEPs provide software services to connect and communicate...

As markets try to look through the blizzard of policy changes flowing out of Washington, the crowd has shifted its preferences considerably in recent weeks based on a sector lens....
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.