
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Axsome Expedites Completion Timeline Of AD Study On AXS-05

Axsome Therapeutics, Inc. (NASDAQ:AXSM) announced that it is accelerating the completion time of the phase II/III ADVANCE-1 study, which currently evaluates AXS-05 for the treatment of agitation associated with Alzheimer's disease (AD). As a result, top-line data from the study is now expected in early second-quarter 2020, which was previously expected in the third.
The randomized, double-blind ADVANCE-1 study is currently enrolling patients who are aged 65 years and above. The company is looking to ensure the safety of the given patient population amid the COVID-19 pandemic as they stand at highest risk for COVID-19-related fatalities. Per the company, the AXS-05 arm along with the placebo arms is fully enrolled and more than 90% of patients is estimated to have completed the study.
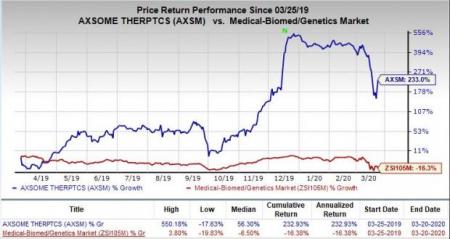
Shares of Axsome have skyrocketed 233% in the past year against the industry’s decline of 16.3%.
AXS-05 is a novel, oral, investigational non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist with multimodal activity under development for treating central nervous system (CNS) disorder. Apart from the ADVANCE-1 study, Axsome is conducting a phase III GEMINI study on AXS-05 for treating major depressive disorder (MDD) and the phase III STRIDE-1 study to address patients with treatment-resistant depression (TRD).
Per the company, positive results from its GEMINI study on MDD along with the previously-completed ASCEND study will be sufficient to support the filing of a new drug application (NDA) for AXS-05 to treat MDD. The NDA is expected to be filed in the fourth quarter of 2020.
Notably, the World Health Organization recently declared the COVID-19 outbreak a pandemic, given the alarming levels of its spread and severity. With global coronavirus outbreak persistently posing a threat to human health, speedy development of vaccines is the need of the hour.
We note that efforts to develop a vaccine for combating the deadly novel coronavirus accelerated in the last couple of weeks. Last week, Moderna, Inc., (NASDAQ:MRNA) dosed the first participant in the phase I study of mRNA vaccine (mRNA-1273) against SARS-CoV-2. Quite a few others like Novavax, Inc. (NASDAQ:NVAX) and Inovio Pharmaceuticals, Inc. (NASDAQ:INO) too are following suit.
We remain upbeat about the developments as several companies along with global authorities are working closely to innovate a treatment as early as possible to eradicate this deadly virus.
Zacks Rank
Axsome currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Free: Zacks’ Single Best Stock Set to Double
Today you are invited to download our latest Special Report that reveals 5 stocks with the most potential to gain +100% or more in 2020. From those 5, Zacks Director of Research, Sheraz Mian hand-picks one to have the most explosive upside of all.
This pioneering tech ticker had soared to all-time highs and then subsided to a price that is irresistible. Now a pending acquisition could super-charge the company’s drive past competitors in the development of true Artificial Intelligence. The earlier you get in to this stock, the greater your potential gain.
Moderna, Inc. (MRNA): Free Stock Analysis Report
Novavax, Inc. (NVAX): Free Stock Analysis Report
Inovio Pharmaceuticals, Inc. (INO): Free Stock Analysis Report
Axsome Therapeutics, Inc. (AXSM): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

As markets try to look through the blizzard of policy changes flowing out of Washington, the crowd has shifted its preferences considerably in recent weeks based on a sector lens....

Nvidia’s muted reaction keeps tech on edge, with chipmakers in focus. Nasdaq’s 20980-21000 support holds—for now. A break could mean trouble. With Nvidia done, GDP today and...

Here’s where I see stocks now: Yes, we’ve got some legitimate concerns as some economic warning signs appear—and run up against the tech-driven optimism that’s powered stocks to...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.