Merck & Co., Inc. (NYSE:MRK) announced that the FDA has granted accelerated approval for the label expansion of its PD-L1 inhibitor, Keytruda, for metastatic small cell lung cancer (“SCLC”) in third- or later-line setting. This approval marks the first label expansion for Keytruda in the SCLC indication.
The approval was based on pooled data from SCLC cohorts of two clinical studies – KEYNOTE-158 (cohort G) and KEYNOTE-028 (cohort C1) – which evaluated the drug in SCLC patients whose disease has progressed after platinum-based chemotherapy and at least one other prior line of therapy. Data demonstrated that treatment with Keytruda achieved an objective response rate of 19% and complete response rate of 2% in the patient population. The duration of response was six months or longer in 94% of the patients, 12 months or longer in 63% of patients and 18 months or more in 56% of patients.
However, continued approval of the drug in this indication will depend on verification and description of clinical benefit in the confirmatory studies.
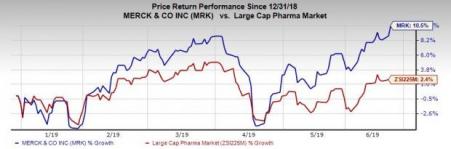
Merck’s shares have risen 10.5% this year so far compared with the industry’s increase of 2.4%.
Keytruda generated sales of $2.27 billion in the first quarter of 2019, up around 5.6% sequentially and 55% year over year. Sales were driven by the launch of indications globally. Keytruda sales are gaining particularly from strong momentum in first-line lung cancer indication both as monotherapy and with the rollout of the chemo combo in both non-squamous and squamous non-small cell lung cancer (“NSCLC”).
Per the press release, SCLC occurs in 10-15% of lung cancer patients. A label expansion in this indication is likely to strengthen Keytruda’s position further in the lung cancer market.
Keytruda is continuously growing and expanding into new indications, treatment setting or geographies and markets globally. Earlier this month, the drug received continued approval as the first-line treatment for recurrent or metastatic head and neck squamous cell cancer Moreover, the drug received approval for multiple label expansions earlier in 2019 including expansion of NSCLC patient population in the United States, Europe and China. A decision on the label expansion of the drug to include first-line treatment of advanced or metastatic renal cell carcinoma (“RCC”) is expected later this week.
However, in 2019, Keytruda faced failure in two clinical studies evaluating it as a second- or third-line treatment of metastatic triple-negative breast cancer or second-line treatment of advanced hepatocellular carcinoma, the most common type of liver cancer.
The Keytruda development program is also progressing well and the drug is being studied for more than 30 types of cancer in more than 900 studies, including more than 600 combination studies. Merck is collaborating with several companies including Amgen (NASDAQ:AMGN) , Incyte (NASDAQ:INCY) , Glaxo (NYSE:GSK) and Pfizer (NYSE:PFE) separately for the evaluation of Keytruda in combination with other regimens.
Several other regulatory decisions for new indications in the United States as well as in Europe are pending in 2019, which, if approved, can further boost sales.
Apart from Keytruda, Merck is also focusing on acquisitions to strengthen its oncology pipeline. The company has offered to acquire three biotech companies so far this year spending more than $2 billion. The company has completed the acquisition of Immune Design, which was announced in February, to boost its capabilities in infectious diseases and cancer. In May, it entered into an agreement to buy Peloton Therapeutics, which will add a late-stage novel late-stage RCC candidate to Merck’s pipeline. Earlier this month, the company announced a definitive deal to acquire Tilos Therapeutics, which has pre-clinical oncology candidates in its pipeline.
Zacks Rank
Merck currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
This Could Be the Fastest Way to Grow Wealth in 2019
Research indicates one sector is poised to deliver a crop of the best-performing stocks you'll find anywhere in the market. Breaking news in this space frequently creates quick double- and triple-digit profit opportunities.
These companies are changing the world – and owning their stocks could transform your portfolio in 2019 and beyond. Recent trades from this sector have generated +98%, +119% and +164% gains in as little as 1 month.
Click here to see these breakthrough stocks now >>
GlaxoSmithKline plc (GSK): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Original post
Zacks Investment Research