
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Roche's Polivy Gets FDA Nod, Gazyva Meets Goals In Phase II

Roche Holdings AG (OTC:RHHBY) announced that the FDA has granted accelerated approval to lymphoma drug, Polivy (polatuzumab vedotin-piiq).
The drug has been approved in combination with bendamustine plus Rituxan (BR) for the treatment of adults with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who have received at least two prior therapies.
Polivy (polatuzumab vedotin-piiq) is an antibody-drug conjugate (ADC), which targets CD79b that utilizes Seattle Genetics’ (NASDAQ:SGEN) technology.
Polivy was developed and will be commercialized by Genentech, a member of the Roche Group.
We remind investors that the approval comes two months ahead of the Prescription Drug User Fee Act (PDUFA) action date of Aug 19, 2019. The Biologics License Application (BLA) was granted priority review as well by the FDA. Polivy was previously granted Breakthrough Therapy designation by the FDA.
While the accelerated approval was granted for this indication, based on complete response rates observed in the phase Ib/II GO29365 study, the continued approval for the same may be contingent upon verification and description of clinical benefit in a confirmatory trial. Results of the study showed that 40% of patients treated with Polivy plus BR achieved a complete response compared to 18% with BR alone.
We note that Polivy was also granted PRIME (PRIority MEdicines) designation by the European Medicines Agency for the treatment of people with R/R DLBCL in 2017.
DLBCL is the most common form of non-Hodgkin lymphoma (NHL), accounting for about one in three cases of NHL. Approximately 150,000 people are estimated to be diagnosed with DLBCL each year globally. Polivy is currently being evaluated for the treatment of several types of NHL.
The approval will broaden Roche’s hematology portfolio, which comprises approved drugs like MabThera/Rituxan, Tecentriq, Gazyva, and Venclexta in collaboration with AbbVie (NYSE:ABBV) . The company also has Hemlibra in its portfolio, a bispecific monoclonal antibody for the treatment of haemophilia A.
Roche is currently looking to diversify its portfolio in wake of stiff competition from biosimilars for key drugs such as Avastin, Rituxan and Herceptin from Novartis and Amgen (NASDAQ:AMGN) .
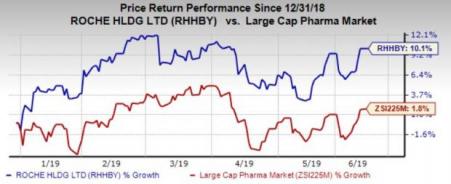
The stock has gained 10.1% in the year so far compared with the industry's 1.8% growth.
Concurrently, Roche also announced positive top-line results from a phase II study, NOBILITY, which is evaluating the safety and efficacy of Gazyva (obinutuzumab) in adults suffering from proliferative lupus nephritis, a severe and potentially life-threatening disorder of the kidneys. The study met its primary endpoint. Results showed that Gazyva in combination with standard of care (mycophenolate mofetil or mycophenolic acid and corticosteroids) demonstrated enhanced efficacy compared to placebo plus standard of care in achieving complete renal response at one year. In addition, Gazyva met key secondary endpoints showing improved overall renal responses (complete and partial renal response) and serologic markers of disease activity as compared to placebo.
Zacks Rank
Roche currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Will you retire a millionaire?
One out of every six people retires a multimillionaire. Get smart tips you can do today to become one of them in a new Special Report, “7 Things You Can Do Now to Retire a Multimillionaire.”
AbbVie Inc. (ABBV): Free Stock Analysis Report
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Seattle Genetics, Inc. (SGEN): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Caesars Entertainment (NASDAQ:CZR), a leading gambling stock, traded around 3% higher on Wednesday morning, though the stock was trading around 1.5% lower shortly before...

Amazon (NASDAQ:AMZN) is making a significant push into the future with a robust investment in robotics and artificial intelligence. The company has earmarked $35 billion for...

Home Depot’s (NYSE:HD) Q4 2024 report and guidance for 2025 have plenty to be unhappy about, but the simple truth is that this company turned a corner in 2024. It is on track for...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.