NBTXR3 clinical trial starts in liver indications
Nanobiotix (PARIS:NANOB) has been granted approval in France to commence pilot clinical development with NBTXR3 for the treatment of liver cancers, allowing the start of a Phase I/II study in line with expected timelines. Liver cancers (including both primary and liver metastases) represent the next indications for NBTXR3, with trials in soft tissue sarcoma (STS), head and neck (H&N) cancer and rectal cancer ongoing. Our valuation is €505m.
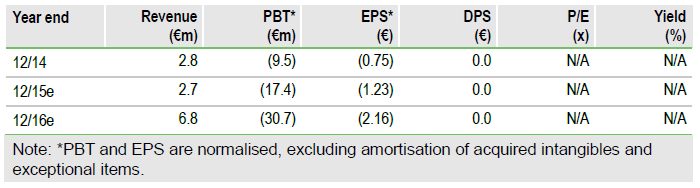
The Phase I/II liver cancers trial will include both primary liver cancer and liver metastases and will explore both intra-lesion and intra-arterial dosing in Phase I, with Phase II in three groups (liver metastases and hepatocellular carcinoma/HCC, both with and without intrahepatic thrombosis) and efficacy measured by response rate, progression-free (PFS) and overall survival (OS), in addition to safety. We believe initial data could become available during 2016. If positive, we assume pivotal development could start in 2017 and forecast initial launches in 2021.
The most common primary liver cancer is HCC, although incidence is much lower in the US and Europe than in Asia (30,000 new cases in the US per year compared to 466,000 in Eastern Asia; source: GLOBOCAN 2012). Liver metastases arise from the spread of other primary cancers (such as colorectal, breast and lung cancer). In colorectal cancer alone there are nearly 150k new cases in the US each year, of which c 50% develop hepatic metastases; hence liver metastases forms the bulk of our €500m US/EU peak liver cancers sales.
To Read the Entire Report Please Click on the pdf File Below
Which stock should you buy in your very next trade?
AI computing powers are changing the stock market. Investing.com's ProPicks AI includes 6 winning stock portfolios chosen by our advanced AI. In 2024 alone, ProPicks AI identified 2 stocks that surged over 150%, 4 additional stocks that leaped over 30%, and 3 more that climbed over 25%. Which stock will be the next to soar?
Unlock ProPicks AI