This week, the biotech sector was in focus with updates from biotechs evaluating their pipeline drugs and vaccines for the treatment of COVID-19. A new strain of coronavirus, which first appeared in late 2019 in China, has become a raging pandemic. This disease has been named COVID-19 and causes severe pneumonia-like symptoms in infected patients.
Recap of the Week’s Most Important Stories:
Novavax Surges on Funding for COVID-19 Vaccine: Shares of late-stage biotechnology company, Novavax, Inc. (NASDAQ:NVAX) , surged after it announced that the Coalition for Epidemic Preparedness (CEPI) awarded an initial funding of $4 million to support its efforts to develop a COVID-19 vaccine. CEPI and Novavax are in discussions regarding additional funding from CEPI to address the costs through the phase I.
Novavax is currently evaluating multiple recombinant nanoparticle vaccine candidates in animal models prior to advancing to clinical trials. The phase I is expected to start in late spring 2020. Novavax’s COVID-19 vaccine candidates were created with its proprietary recombinant protein nanoparticle technology platform to generate antigens derived from the coronavirus spike (S) protein. The company also expects to utilize its proprietary Matrix-M adjuvant with its COVID-19 vaccine candidates to enhance immune responses. Coronaviruses are a large family of viruses that spread from animals to humans and include diseases such as Middle East Respiratory Syndrome (MERS) and SARS, in addition to COVID-19.
Mesoblast Surges on News of Coronavirus Study: Mesoblast Limited (NASDAQ:MESO) announced plans to evaluate its allogeneic mesenchymal stem cell (MSC) product candidate, remestemcel-L, in patients with acute respiratory distress syndrome (ARDS) caused by coronavirus (COVID-19) in the United States, Australia, China and Europe. Shares soared on the news. Mesoblast is actively discussing with various government and regulatory authorities, medical institutions and pharmaceutical companies to implement these activities. Remestemcel-L is currently being studied in numerous clinical trials across several inflammatory conditions in elderly patients with lung disease and adults and children with steroid-refractory acute graft versus host disease.
Can-Fite to Evaluate RA Drug for Coronavirus: Can-Fite BioPharma Ltd. (NYSE:CANF) is exploring a collaboration to evaluate the effect of lead candidate, piclidenoson, against coronavirus. Piclidenoson, a novel, first-in-class, A3 adenosine receptor agonist (A3AR), is currently in phase III studies for rheumatoid arthritis (RA) and psoriasis. Data from the interim analysis of the phase III study will be released during the fourth quarter of 2020. This move is driven by reports of RA drugs being introduced for the treatment of coronavirus. Shares of the company rallied significantly on this news. Can-Fite’s drug candidates possess anti-viral effect protected by a U.S. patent, US7589075.
Gilead Announces Positive Data on Descovy: Gilead Sciences, Inc. (NASDAQ:GILD) announced positive long-term results from the DISCOVER study on Descovy for pre-exposure prophylaxis (PrEP). These longer-term efficacy and safety outcomes from the DISCOVER study showed that Descovy is effective for HIV prevention with non-inferior efficacy to Truvada and the drug has an improved bone and renal safety profile compared with Truvada at week 96. Meanwhile, a separate analysis of the DISCOVER study demonstrated that Descovy and Truvada were effective and well tolerated in Black and Hispanic/Latinx participants.
AIM ImmunoTech Drug Being Tested for COVID-19: AIM ImmunoTech (NYSE:AIM) announced that the National Institute of Infectious Diseases (NIID) in Japan will begin testing its drug, Ampligen, as a potential treatment for COVID-19, the new coronavirus infectious disease caused by SARS-CoV-2. The experimental program will be conducted at both the NIID and the University of Tokyo.
Kala Announces Data on Dry Eye Disease Drug: Kala Pharmaceuticals, Inc. (NASDAQ:KALA) announced positive top-line results from a late-stage study, STRIDE 3. The study is evaluating KPI-121 0.25%, which Kala plans to commercialize under the brand name EYSUVIS (loteprednol etabonate ophthalmic suspension) 0.25%, for the treatment of dry eye disease. STRIDE 3 met both of its primary efficacy endpoints, demonstrating a statistically significant improvement in the symptom endpoint of ocular discomfort severity at day 15 in the overall intent-to-treat (ITT) population and the predefined subgroup of ITT patients with more severe ocular discomfort at baseline.
Kala currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Performance
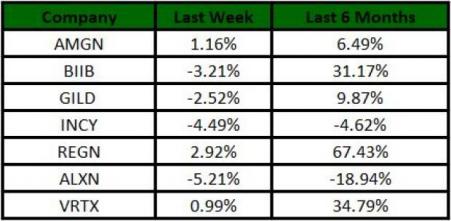
The Nasdaq Biotechnology index lost 3.41% in the last five trading sessions. Among the biotech giants, Regeneron gained 2.92% during this period. Over the past six months, shares of Regeneron have gained 67.43%. (See the last biotech stock roundup here: Biotech Stock Roundup: GILD to Buy Forty Seven, Pipeline Updates From BIIB & More)
What's Next in Biotech?
Stay tuned for more pipeline updates with a focus on treatments for the novel COVID-19.
The Hottest Tech Mega-Trend of All
Last year, it generated $24 billion in global revenues. By 2020, it's predicted to blast through the roof to $77.6 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Novavax, Inc. (NVAX): Free Stock Analysis Report
Can-Fite Biopharma Ltd (CANF): Free Stock Analysis Report
Hemispherx BioPharma, Inc. (AIM): Free Stock Analysis Report
Mesoblast Limited (MESO): Free Stock Analysis Report
Kala Pharmaceuticals, Inc. (KALA): Free Stock Analysis Report
Original post
Zacks Investment Research